T1D Guide
T1D Strong News
Personal Stories
Resources
T1D Misdiagnosis
T1D Early Detection
Research/Clinical Trials
COUR Pharma Pursues Disease-Modifying Therapy for T1D
COUR Pharma’s CNP-103 is a first-of-its-kind tolerogenic therapy designed to retrain the immune system in newly diagnosed type 1 diabetes—stopping islet cell destruction, preserving insulin, and potentially reversing abnormal glucose levels. T1D Strong spoke with Chief Medical Officer, Dr. Paul Peloso, about this groundbreaking approach.
.jpg)
CNP-103 is among the first disease-modifying tolerogenic therapies currently in clinical testing. Here, Dr. Paul Peloso explains how it works, what they expect from the trial, and the innovative benefits tolerogenic therapies could bring to people with autoimmune conditions like type 1 diabetes (T1D).
Paul Peloso’s Backstory
Dr. Paul Peloso joined COUR in 2024 as Chief Medical Officer, where he leads the company’s clinical development programs. Peloso has a long history in the biopharmaceutical industry, holding senior leadership roles at Horizon Therapeutics, AbbVie, and Merck.

Throughout his career, he has led programs in rheumatology and rare diseases and contributed to the global development of numerous innovative therapies. Before joining COUR, Peloso served as the Chief Medical Officer for Acelyrin, a biopharmaceutical company specializing in immunology therapies for immune-mediated diseases.
In addition to his work in biotech advancing treatments for autoimmune disorders, Peloso has a personal connection to type 1 diabetes: his son was diagnosed at age 12. As a father to a child with type 1 diabetes, Peloso speaks from experience: “When you have type 1 diabetes, your entire family has type 1 diabetes.”
He’s optimistic about therapies in development, noting a wave of research focused on tolerance. “Something is going to work,” he said. “I’m enthusiastic about the future—not only do we have tools like CGMs (continuous glucose monitors) and advanced insulins to better manage diabetes, but also therapies that preserve beta cell function in healthy ways, without shutting down other parts of the immune system.”
Enter COUR Pharma’s CNP-103
About COUR Pharma
COUR Pharmaceuticals, a clinical-stage biotechnology company, was founded in the early 2010s to treat patients with autoimmune diseases. COUR develops disease-modifying therapies to induce antigen-specific immune tolerance treatments for autoimmune diseases like type 1 diabetes, celiac disease, myasthenia gravis (MG) and primary biliary cholangitis (PBC).
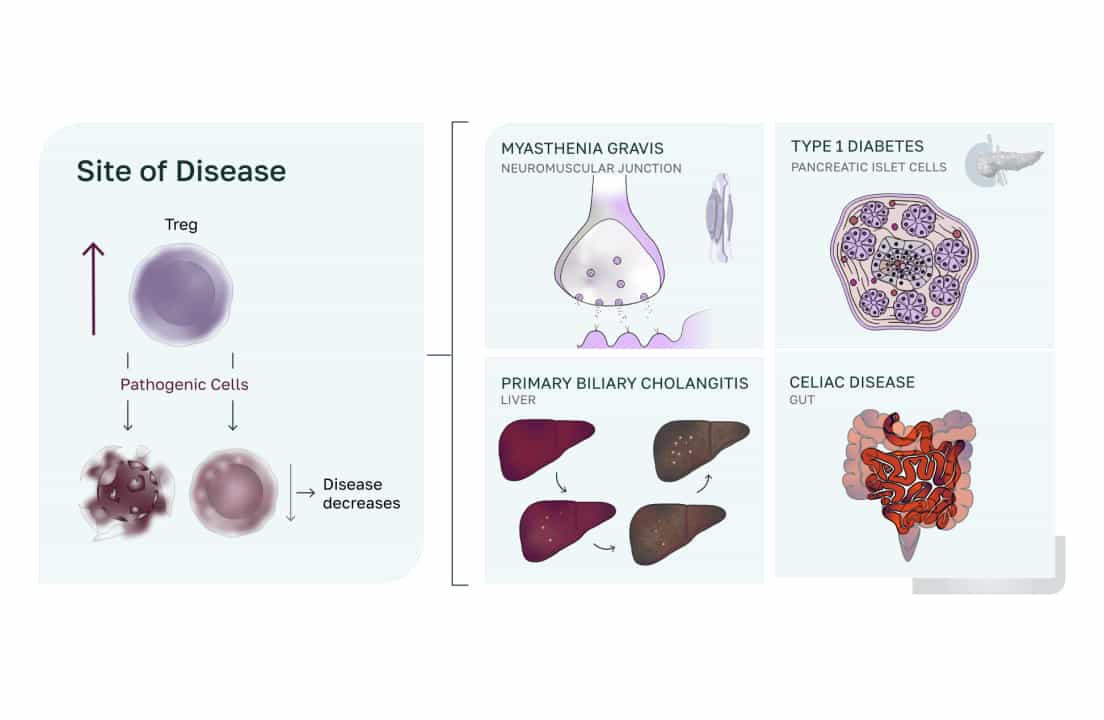
COUR aims to treat autoimmune diseases (not simply suppressing symptoms) by targeting the primary immune dysfunction.
What is Immune Tolerance?
COUR nanoparticles (CNPs) create antigen-specific immune tolerance, which can be described as a reprogramming of the immune system to stop attacking specific self-antigens while leaving other immune functions intact. Peloso explained how the immune system normally tolerates the body’s own cells and how this process can go awry in autoimmune diseases, such as T1D, after a virus and environmental exposures are introduced.
“Normally, our bodies break down about a billion cells every day, and these dead cells, called apoptotic bodies, need to be safely processed. These apoptotic bodies travel through the bloodstream and are usually handled by the liver and spleen.”
In type 1 diabetes and other autoimmune conditions, something tricks the body into seeing these normal proteins as harmful. The immune system gets confused and attacks the body’s own cells. He added that these special immune cells, known as antigen-presenting cells, consume these dead cells and display fragments of their proteins on their surface. This is how the immune system learns: it recognizes these proteins as part of the body, so it doesn’t attack them.
“People are looking at a variety of different approaches,” said Peloso, “but this tolerance approach is the next frontier in medicine.”
COUR Pharma’s Tolerogenic Therapy—CNP-103
In February 2025, the FDA cleared the biotech’s Investigational New Drug (IND) application for CNP-103, a nanoparticle in development for type 1 diabetes. Then, on August 19, 2025, COUR Pharma began trial enrollment for this investigational tolerogenic therapy, now in Phase 1b/2a, and dosed its first patient with CNP-103.
How it Works
The treatment, also known as antigen-specific immune tolerance, delivers four key diabetes-related antigens — preproinsulin, GAD65, IGRP, and ZnT8 —to patients in an effort to halt the immune response that causes type 1 diabetes.
“CNP-103 mimics the body’s natural mechanism to teach the immune system not to attack itself. This drug works by tapping into this normal process that has been happening in the human body for as long as humans have existed.”
CNP-103 is a biodegradable nanoparticle encapsulating these four key proteins that cover >95% of the known autoantigens driving T1D. “The nanoparticle is made from lactic and glycolic acid, substances already found in the body,” Peloso said. “These materials have been safely used in medicine and cosmetics for more than 20 years.”
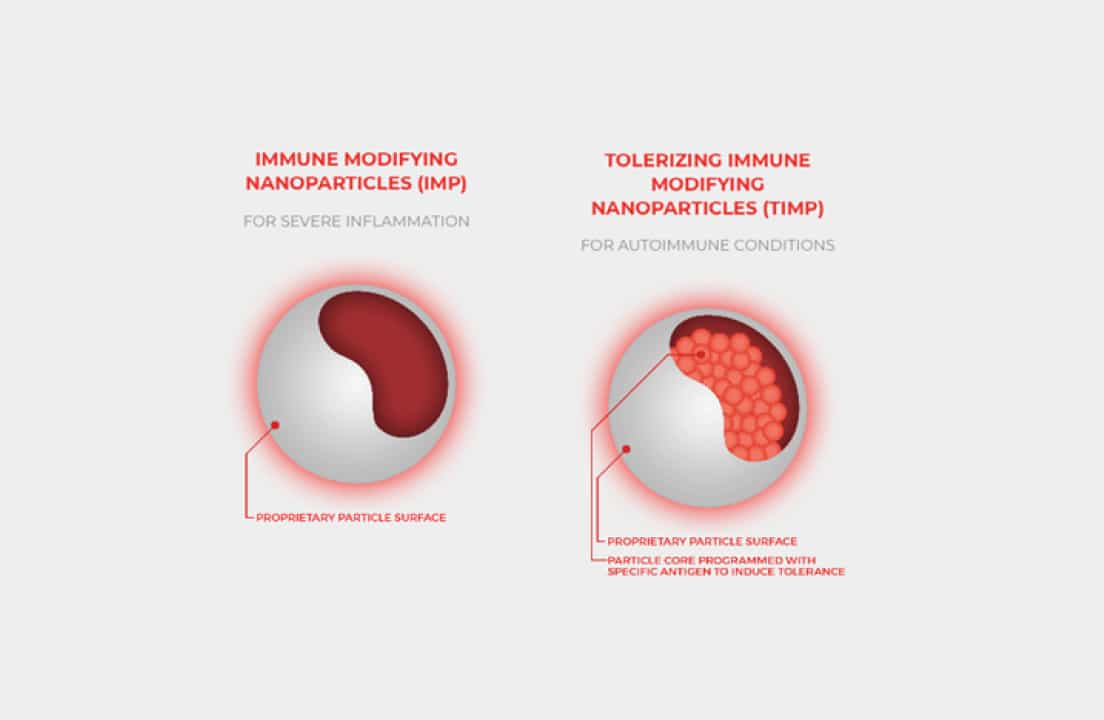
By inducing tolerance to these proteins, COUR seeks to prevent further islet cell destruction and promote antigen tolerance, while teaching the immune system to stop attacking the insulin-producing beta cells. “Once inside the body, the particles are broken down, and special immune cells called antigen-presenting cells process them, showing the immune system these antigens.”
One of the goals of CNP-103 is for people recently diagnosed with T1D to maintain some natural insulin production by preserving the body’s remaining insulin-producing beta cells in the pancreas. This is gauged by measuring C-peptide levels (a marker of beta-cell function).
No Immunosuppression Needed
This tolerogenic reprogramming therapy differs from traditional treatments that rely on immunosuppressant drugs, which shut down immune activity and leave patients vulnerable to infections and cancers. In contrast, CNP-103 leaves the immune system intact.
“Another point of differentiation to highlight is that with some therapies, you need to give another drug to suppress the immune system; no such treatment is required for us,” said Peloso. “Because we’re embedded in this normal pathway, the body’s sort of preprogrammed to recognize all of this as self. We’re actively using this standard pathway, so there’s nothing to turn off that’s not behaving well apart from the lack of tolerance to self.”
CNP-103 Phase 1b/2a Trial
The Phase 1b/2a trial will examine both safety and the ability of CNP-103 to maintain C-peptide levels. In the new trial, patients receive an IV infusion of the therapy.
“It’s given intravenously, and its tiny particles are about the same size as the body’s naturally circulating apoptotic bodies, which are negatively charged,” said Peloso. “This allows the drug to fit into the body’s normal cellular cleanup system. We could debate whether the intravenous method is good or bad—on the good side, you can be sure that you get the drug, on the bad side, it does require a visit to an office.”
Peloso added that the early signs are well-received. “They get two IV infusions at baseline, one at day one and the second one on day eight; it doesn’t have to be exactly on day eight, just a week later. Across the program, we’re looking at two to three boosters per year. So, about four injections per year.”
Peloso noted that they are also working on an oral technology, which is in its early stages of development.
CNP-103 Phase 1a/2b Trial Details
- Participants: Ages between 12 and 35 years old
- Recently Diagnosed: Type 1 diabetes diagnosed within the last 6 months (stage 3)
- Additional criteria: Must have a stimulated C-peptide (measure of residual insulin production) of >0.2 nmol/L
- Design: Randomized, placebo-controlled and double blind, meaning neither the participant nor the researchers will know who is receiving the actual treatment.
- Duration: Participants will be followed for about 12 months after dosing to assess safety and whether C-peptide levels are preserved.
- Dosing: IV infusion(s)
“We’ve had this technology evaluated in 150 patients so far, and it’s been extremely well tolerated,” Peloso said. “When I talk to some of the principal investigators, they often ask me, ‘Do we get worrisome infusion reactions?’ It’s been seen in other therapies but not ours yet.”

Success in this trial would mean:
- The therapy is safe and tolerated (no serious immune-related side effects).
- Patients retain more C-peptide compared to placebo after 12 months. (This tells how much insulin the pancreas is still making.)
- Individuals tend to exhibit trends toward lower insulin needs, improved HbA1c levels, and fewer episodes of hypoglycemia.
“It’s a relatively simple test called the mixed meal tolerance test, a standardized mixture of sugar, protein and fat,” Peloso said. “We measure blood glucose, and we measure C-peptide levels.”
“We’re looking at a marker of insulin production, so the beta cells produce insulin, but if you go back one step, they actually produce pro-insulin, which is broken into C-peptide and insulin. That C-peptide is something you can measure. It’s a very direct measure of pancreatic function.”
During the trial, participants need to continue taking insulin.
Why Stage 3 Type 1 Diabetes?
CNP-103 is unique in that it targets patients within six months of diagnosis (stage 3), a group that currently has no FDA-approved treatments to modify the autoimmune condition. Currently, Teplizumab (Tzield) is the only FDA-approved disease-modifying therapy designed to support patients in stage 2 of type 1 diabetes, while other therapies remain under investigation.
Stages of Type 1 Diabetes:
- Stage 1: Presence of two or more diabetes-related autoantibodies with normal blood glucose levels.
- Stage 2: Abnormal blood glucose levels without clinical symptoms.
- Stage 3: Clinical diagnosis of type 1 diabetes with symptomatic hyperglycemia.

Peloso said they’ve spoken to leading experts, and there’s a lot of enthusiasm for implementing the therapy for stages 1 and 2. He explained a few practical reasons they started with stage 3. “From a benefit and risk point of view, it’s just easier to take patients who have a defined need, and the risk tolerance is higher.”
“Stage 2, they’re generally healthy, but they’re not in the same situation as stage 3 patients; there’s a bit better payoff for benefit and risk,” he added. Additionally, during the honeymoon phase (stage 3), the pancreas is still capable of producing some insulin on its own. “If we reduce stress on the beta cells—by giving insulin so the beta cells don’t have to work too hard, and through diet, exercise, and stopping the immune attack—we hope to keep that healthy insulin production going for longer.”
Also, as a small biotech, it makes more business sense. “What we know from the Tzield studies, let’s just say it takes two and a half to three years, to get to a point where you can understand if the drug is working and for us—since we’re a small biotech, in a year we can figure it out in stage 3, as compared to taking three years to figure out if it is working in stage 2.”
From Inflammation to Long-Term Beta Cell Health
“The challenges, as you might expect, are that in order to identify patients who we can track the decline in a certain way, they have to be above a certain level of C-peptide—that means within six months of the disease,” said Peloso. “I hope we can stretch this out to a year. We’re looking to see robust evidence that the drug is working. Overtime, we hope it will expand.”
As Peloso mentioned, “Our ultimate goal is to reverse the decline of beta cell destruction, and once we turn off the inflammation, and we have a stable enough level of beta cell function, the hope would be that it would be sufficient forever.”
Could CNP-103 Work with Established T1D Individuals?
As CNP-103 shows potential for people with T1D across stages 1 through 3, it may also help later-stage patients when used in conjunction with islet cell transplants.
“It’s amazing there have been so many investments in T1D, and it’s amazing to see how my son’s experience has changed. And now people are talking about beta cell transplants,” Peloso said. “So, this potentially is the technology we could give in concert with transplant in people with long-standing disease, so they get the beta cell function, and the immune system doesn’t reattack the beta cells.”
COUR Pharma’s Distinct Approach
Peloso described the uniqueness of COUR Pharma’s approach to type 1 diabetes and how they’re tapping into the body’s natural tolerance system. The therapy trains your body to use the same process it already uses to prevent the immune system from attacking itself.
“This ancient immune pathway has been used for millions of years to recognize its own cells,” said Peloso. “And the treatment uses the liver and spleen, organs that help process the therapy safely.”
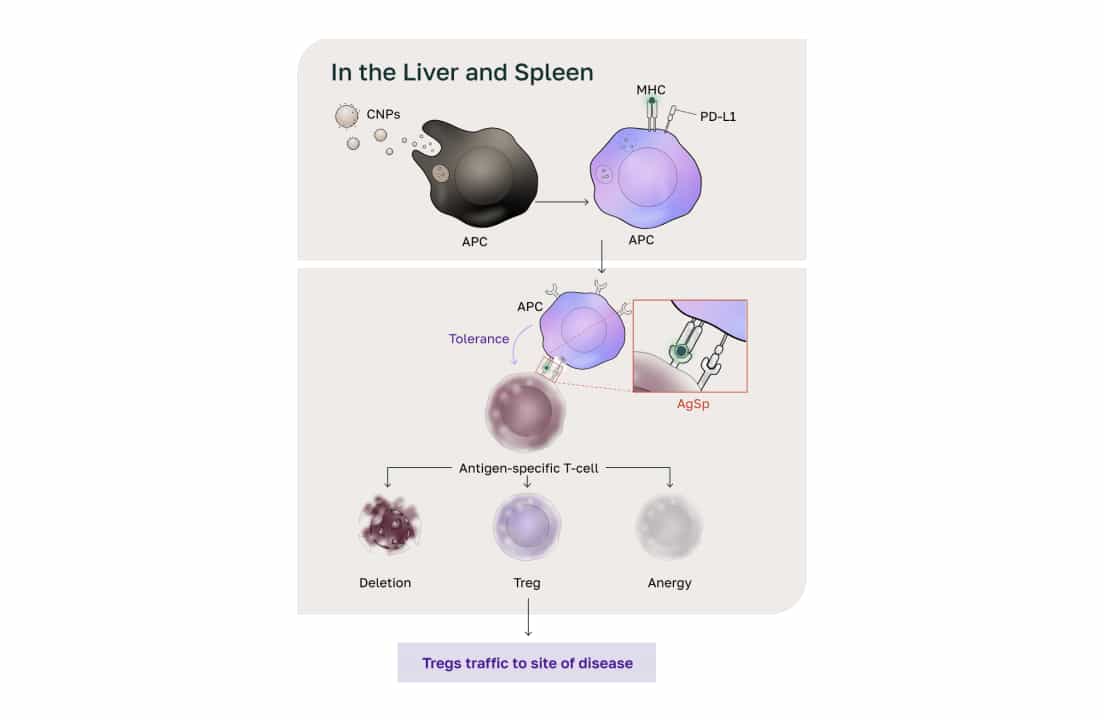
Peloso added that COUR’s approach isn’t limited by protein size or genetic subtype. “We have four proteins that collectively cover 95 percent of the known antigens in type 1 diabetes. And so, we’re not limited because of the technology itself. Some platforms are specific for certain genetic subtypes and can only deliver a certain size of protein, but we have no limits in that regard.”
In summary, Peloso describes three key elements of the CNP-103 therapy:
- CNP-103 works with the body’s normal process for handling dead cells and teaches the immune system not to attack itself.
- The therapy is designed as tiny, negatively charged particles that the body recognizes, similar to naturally occurring dead cells.
- There are no restrictions on the amount of antigens (key protein “triggers”) given to a patient, so no special testing is required for eligibility for the therapy.
Saving Alpha Cell Function
Peloso explained that in preclinical models, CNP-103 not only stops inflammation in beta cells but also in alpha cells, which detect and respond to blood glucose levels.
“With current glucose monitors, preserving alpha cell function could help the body manage lows more naturally,” he said.
Alpha cell loss typically occurs later, often a decade into T1D, as a result of ongoing inflammation. “I dream of a future where we can stop this destruction and maintain alpha cell health over time,” Peloso added.
An Optimistic Roadmap Forward
Moving forward, COUR Pharma plans to drive patient awareness and engagement. If CNP-103 Phase 1b/2a trial results are promising, larger Phase 2/3 trials would follow. The next steps are to train the clinical sites and ensure they are prepared to understand the technology, accept patients, and administer the product.
“The question becomes: if you’re successful, how do you maximize getting therapy to all the patients who might be eligible? This is where the bigger companies really excel,” Peloso noted.
“We feel very good about our ability to manage the clinical trial programs to get to FDA approval. Getting to all the patients globally who could benefit once we are approved would potentially require partners.”
COUR Pharmaceuticals is currently enrolling participants in its Phase 1b/2a clinical trial for CNP-103.
For more information or to find a participating site near you, visit COUR Pharma’s clinical trial page.


.webp)





.webp)

.jpg)
.jpg)
.jpg)
.jpg)
.jpeg)
.jpg)




.jpg)

.jpg)

.jpg)
.jpg)


.jpg)


.jpg)
.jpg)
.jpg)
.jpg)

.jpg)


.jpg)


.jpg)












.jpg)










%20(1).png)


















.jpg)















.webp)