T1D Guide
T1D Strong News
Personal Stories
Resources
T1D Misdiagnosis
T1D Early Detection
Research/Clinical Trials
Abbott's Biowearable: One Sensor for Glucose and Ketones
Abbott is developing a new biowearable sensor that may change the way people living with type 1 diabetes manage their health. This sensor will monitor both blood glucose and ketone levels in real time.
.jpg)
The sensor is built on the same platform as the FreeStyle Libre 3, which is the smallest and thinnest continuous glucose monitor (CGM) available. This means it will be compact, wearable, and designed for comfort.
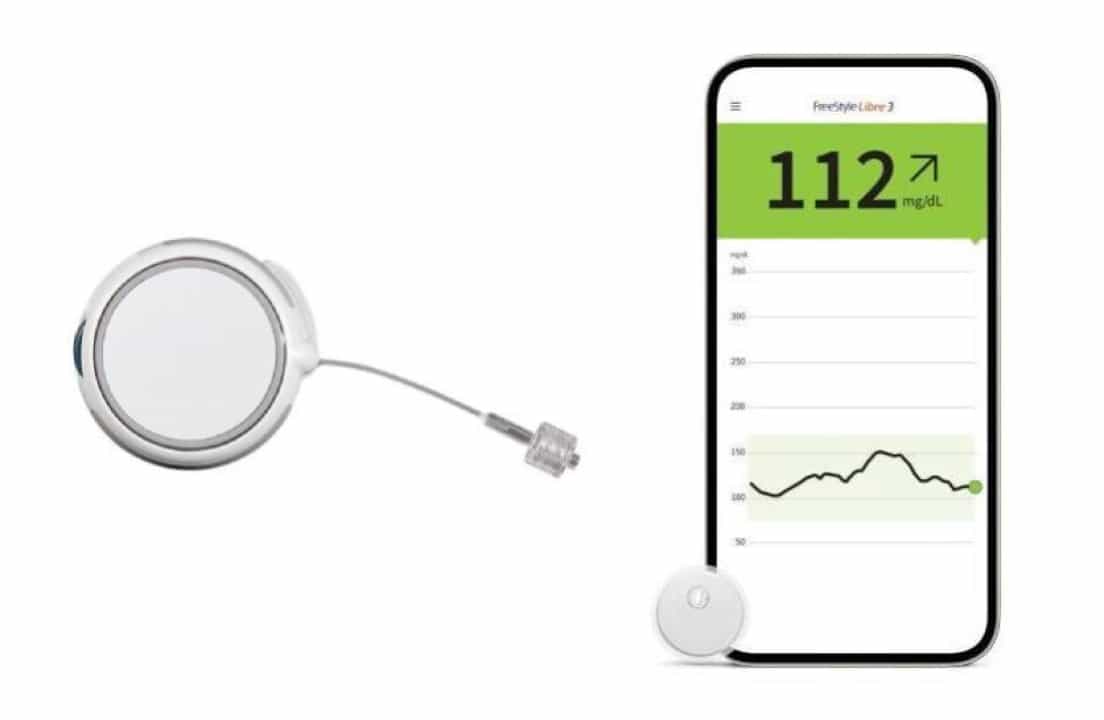
Why Ketone Monitoring Matters
For people living with type 1 diabetes (T1D), ketone monitoring is critical. Ketones are chemicals made by the liver when the body burns fat for energy. When insulin levels are too low, ketones can rise to dangerous levels, leading to diabetic ketoacidosis (DKA).
You can read more about ketones and DKA here.
The Dangers of DKA
DKA, caused by an overload of ketones present in the blood, can lead to diabetic coma or even death.
When the body’s cells don't get the glucose they need for energy, the body begins to burn fat instead. This produces ketones, which are acidic. Normally, insulin helps glucose enter cells, but when there isn’t enough insulin, the body switches to fat for energy, triggering ketone production. As ketones build up in the blood, they make it more acidic and toxic.
The excitement around this sensor isn’t just about tech. It’s about peace of mind. Having real-time ketone data may ease anxiety and help people act fast before DKA becomes a crisis.

High levels of ketones can poison the body. When levels get too high, you can develop DKA. DKA may happen to anyone with diabetes, though it is rare in people with type 2.
Symptoms of DKA include:
- Nausea or vomiting
- Abdominal pain
- Fruity-smelling breath
- Rapid breathing
- Fatigue or confusion
Treatment for DKA usually takes place in the hospital. But you can help prevent it by learning the warning signs and checking your urine or blood regularly.
Ketones can develop for many reasons, not just from a lack of insulin. Illness, dehydration, missed meals, certain medications, or stress can all trigger ketone production.
What’s surprising to many is that ketones can be present even when blood sugar levels appear normal. This is not widely known, and it can delay necessary action. This phenomenon-often referred to as euglycemic DKA or normoglycemic DKA-is rare but dangerous. It occurs when the body produces excess ketones despite blood glucose levels being in or near the normal range.
This new Abbott sensor aims to change that by combining glucose and ketone tracking in one device, offering a clearer picture of what’s happening inside the body.
FDA Breakthrough Device Designation
This breakthrough technology received the "Breakthrough Device" designation from the FDA, which means it qualifies for a faster review process due to its potential to save lives and improve care.
The FDA grants this status to devices that provide more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions. It’s a strong signal that this technology could significantly impact public health and address unmet needs in diabetes care.
A Game-Changer for Type 1 Diabetes
The new Abbott sensor is designed for people living with type 1 diabetes, who are at risk for DKA. It has the potential to alert users to ketone spikes early, before symptoms become serious.
In addition, the sensor will offer all the features people love in the FreeStyle Libre 3:
- Small and discreet design
- No need for finger sticks
- Real-time data and alerts

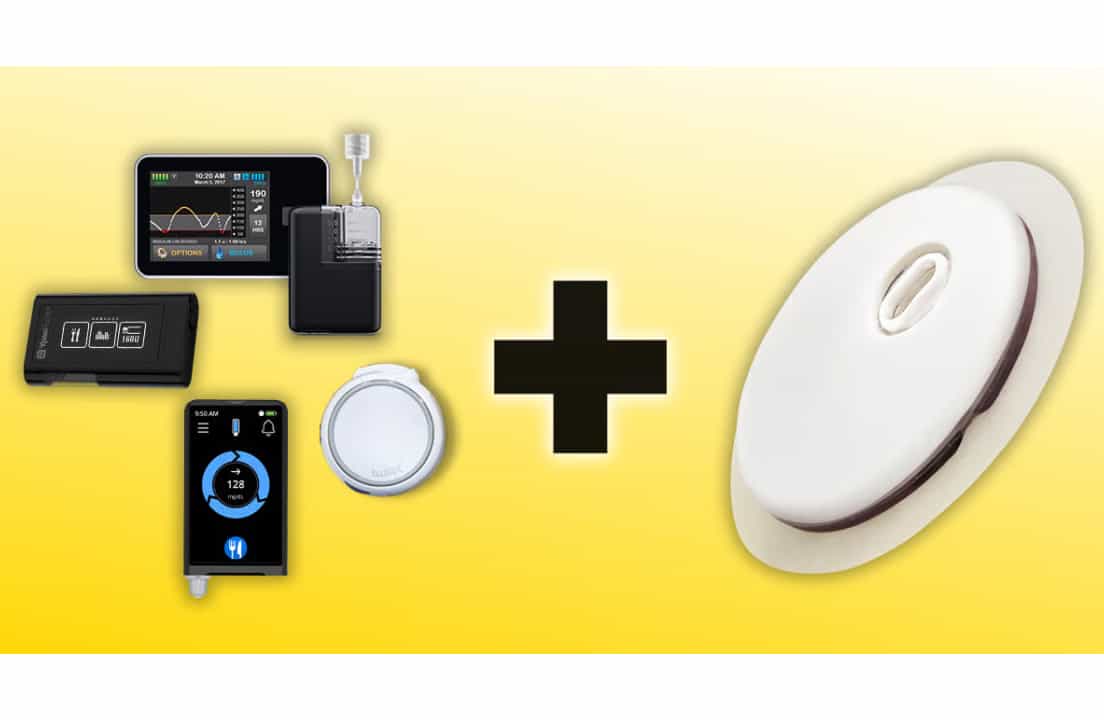
Industry Excitement and Partnerships
Abbott shared updates about the dual-sensor at the 2025 ADA Scientific Sessions. Several insulin pump makers announced they will connect their products to the new sensor.
These include:
- Sequel Med Tech (Twiist pump)
- Tandem Diabetes Care
- Beta Bionics
- Ypsomed and CamDiab
These partnerships will make it easier for people to track both glucose and ketones while using automated insulin delivery systems.
Built for Ease and Accuracy
Chris Scoggins, executive vice president of diabetes care at Abbott, confirmed that the new sensor will use the Libre 3 Plus form, which includes the following updates:
- Improved accuracy
- Updated alerts
- No need to scan for readings
- A single app for all Libre generations
Alarm Fatigue
Alarm fatigue happens when people with diabetes get overwhelmed by frequent alerts from their continuous glucose monitors and insulin pumps.
The Abbott sensor will also allow users to silence alerts for up to six hours. Optional alarms can quietly alert users to high or low glucose, and eventually, high ketones. This is a plus, as many experience alarm fatigue when using diabetes devices.

These alarms can be helpful but may also disrupt sleep, cause stress and lead users to ignore or turn them off. Experts recommend adjusting alert settings, using vibration mode and talking to a health care provider to find a safer balance.
There are glucose companion devices available on the market that can help reduce alarm fatigue and improve diabetes management.
Backed by Research and Trials
Pivotal trials for the new sensor took place in 2023. Regulatory review is next, and Abbott expects to release the sensor commercially in 2025.
What the ADA Says
The American Diabetes Association (ADA) supports using automated insulin delivery (AID) systems.
Some of which offer:
- Reduce glucose highs and lows
- Improve time in range
- Lower the burden of daily diabetes care
This new dual-sensor will fit into that AID ecosystem, adding an extra layer of safety with ketone alerts.

Final Thoughts
The future of diabetes tech is getting smarter and safer. With continuous ketone monitoring, the next wave of CGMs will not only help people manage their daily blood sugars but may also prevent dangerous complications like DKA.
That’s good news for the 1.4 million people living with type 1 diabetes in the U.S. today—and their caregivers, families, and care teams.


.jpeg)





.webp)

.jpg)

.jpg)
.webp)

.jpg)
.jpg)
.jpg)

.jpg)

.jpg)

.jpg)
.jpg)



.jpg)



.jpg)




.jpg)

.jpg)

.jpg)
.jpg)




.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)

.jpg)
.jpg)

.jpg)



.jpg)
.jpg)
.jpg)

.jpg)

.jpg)














.jpg)


.jpg)







.webp)











.webp)











.webp)