T1D Guide
T1D Strong News
Personal Stories
Resources
T1D Misdiagnosis
T1D Early Detection
Research/Clinical Trials
A New Era in Type 1 Diabetes Safety: Continuous Ketone Monitoring
Although continuous ketone monitoring (CKM) remains an emerging practice (no FDA-approved CKM is yet available for diabetes care), experts agree that it holds significant promise for people with type 1 diabetes. By offering around-the-clock ketone tracking and real-time feedback, CKM could help individuals better understand how their bodies respond to illness, stress, food, and exercise, while detecting rising risk earlier.
.jpg)
The practice of ketone monitoring and the technology behind it have evolved rapidly. What once required urine test strips and later finger-stick blood tests is now moving toward wearable sensors that continuously measure ketones in the body, similar to continuous glucose monitoring (CGM).
Most importantly, experts say CKMs could be a game changer for detecting the life-threatening condition of diabetic ketoacidosis (DKA). The goal is also to make the device accessible for those at a higher risk of serious illness, like type 1 diabetes (T1D) individuals with the additional challenge of insulin sensitivity, glucose intolerance, dysglycemia and hypoglycemic unawareness.
How CKM Works
Continuous Ketone Monitoring tracks ketone levels in the body through a small subcutaneous sensor, similar in size and placement to a CGM. The CKM is inserted just under the skin with integrated alarms for high ketone levels.
CKM uses an enzymatic process to measure betahydroxybutyrate (BHB) every few minutes. Additionally, the sensor stores data for later transfer and deciphering.
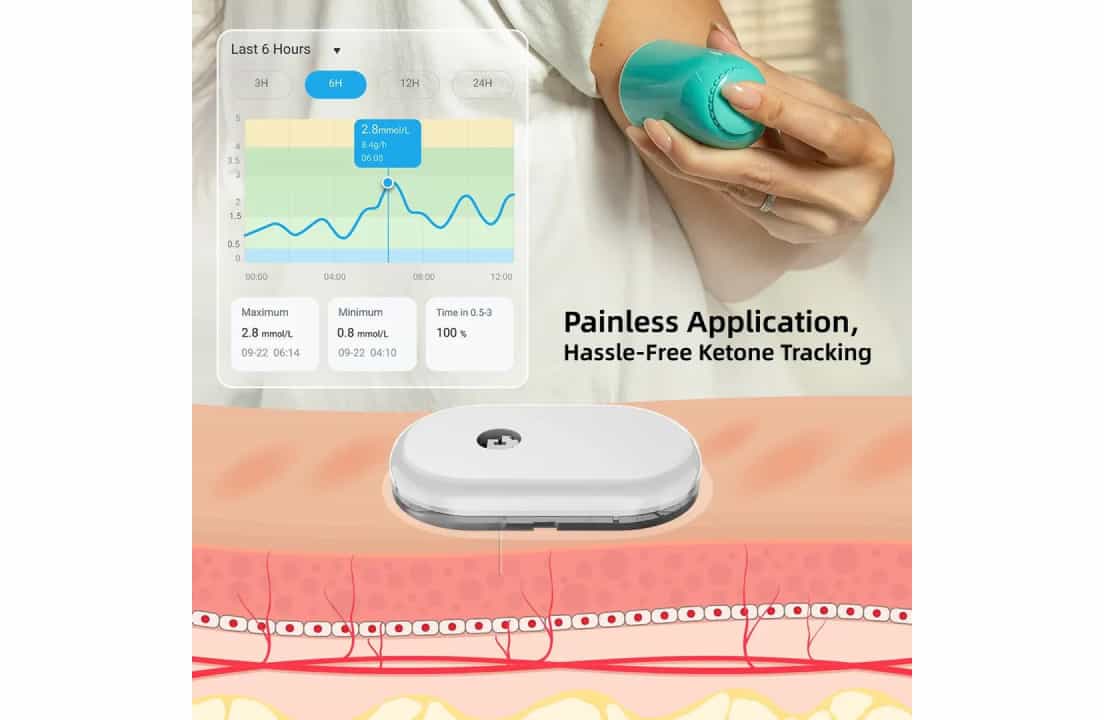
The Greatest Threat: DKA
When cells lack insulin, they burn fat, producing acidic ketones. These high ketones lead to DKA, where individuals may experience confusion, extreme thirst and lethargy. This side effect can be fatal.
Important Fact: DKA is not always detected by high blood glucose levels. Euglycemic DKA is DKA when blood sugar levels aren’t extremely high.
I recently came across a heartbreaking post on Facebook from a mother whose son died from DKA while away at college. He lived alone in his dorm and had always been diligent about managing his diabetes. During a short break from his insulin pump and CGM, he became ill.
He called out of work but didn’t alert his family. When his mother couldn’t reach him, she contacted the school—but by the time they intervened, it was too late.
CKMs Provide Extra Peace of Mind
In addition to providing early warning signs of illnesses such as the flu, CKMs can aid in pump failures or when using certain medications (SGLT-2 inhibitors), enabling quicker intervention than traditional spot checks.
Who Benefits from Wearing CKMs
- Recurrent DKA: T1Ds with a history of frequent ketone buildup.
- Illness (“Sick Days”): Acute illness significantly increases the risk of DKA.
- Intense Exercise: High-intensity workouts can trigger ketone production.
- Insulin Pump Users: Especially prone to rapid insulin deprivation if the pump fails.
- Pregnancy: Pregnant individuals with diabetes have a period of heightened risk for DKA.

- SGLT-2 Inhibitor Users: These diabetes medications can increase the risk of DKA.
- Metabolic health: People trying to lose weight.
- Low-Carb/Keto Diets: Individuals on a ketogenic low-carb diet can identify what consistently keeps them in ketosis and spot foods and habits that keep them out. You can see how food choices affect your fat burning.
CKMs – What’s Available and What’s Coming
Available or Near-Available Devices
1. SiBio KS1 Continuous Ketone Monitoring (CKM)
- A wearable device that provides real-time ketone trend tracking (ketone readings every few minutes via Bluetooth).
- Often marketed to people following low-carb or keto lifestyles.
- Not FDA-cleared as a medical device for diabetes management in the U.S., and availability may vary by region.

2. Consumer CKM Devices
- Several wearable products (e.g., listed on CKMonitors or Ketonu sites) claim to continuously track ketones for wellness and metabolic awareness.
- These devices are not FDA-approved medical devices and are marketed for general wellness rather than clinical use.
Abbott's Lingo system is an over-the-counter CGM platform designed for general health and wellness rather than diabetes management. It consists of the wearable biosensor and a companion smartphone app to help users understand how diet, exercise and stress affect blood sugar levels in real-time.
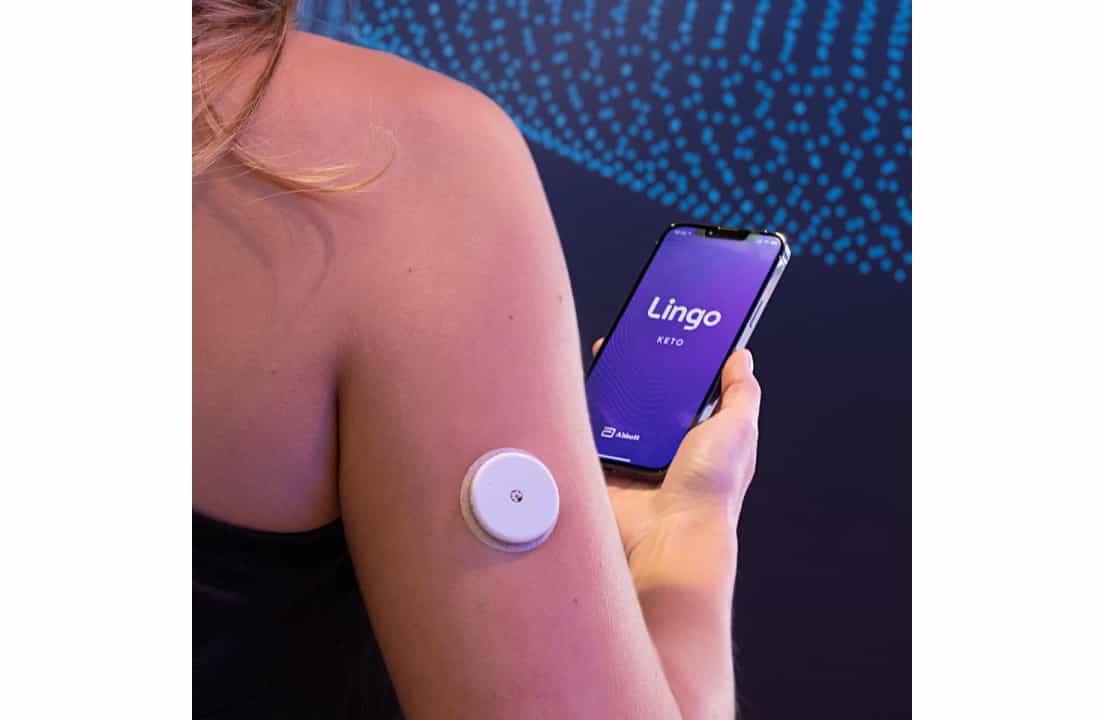
Devices Under Development or Coming Soon
While wearing a CGM, insulin pump, and CKM can feel burdensome, the protection they provide during illness can be lifesaving. What's more, several CKM sensors under development are designed for glucose and ketone monitoring simultaneously.
1. Abbott Dual Glucose-Ketone Sensor (under development)
- Abbott (maker of FreeStyle Libre CGMs) is actively developing a single sensor that continuously monitors both glucose and ketones.
- It has a Breakthrough Device designation from the FDA, which can speed up review.
- Expected to pair with insulin pumps and CGM apps for real-time ketone and glucose data, improving early detection of DKA risk.
- Multiple insulin pump makers (e.g., Tandem, Beta Bionics, Ypsomed, Sequel Med Tech) are planning integration with this sensor once it’s approved.
2. Integrated Continuous Glucose + Ketone Monitoring Platforms (early development)
- Startups such as Integrated Medical Sensors (IMS) are developing multiplex sensors to monitor glucose, ketones, lactate, and other metrics from a single wearable patch.

3. Research Prototypes (Microneedle Arrays & Advanced Biosensing)
- Academic and industry research projects are exploring microneedle electrochemical sensors that could continuously measure ketones (BHB) alongside glucose. These are pre-commercial and in validation/testing phases
The Next Line of Defense Against DKA
The Lancet reports, “The ability to reduce the risk of developing DKA remains a major care gap for people with diabetes, particularly those on intensive insulin therapy. The anticipated availability of continuous ketone monitoring (CKM) has the potential to reduce the risk of developing DKA, one of the most life-threatening acute complications of type 1 and type 2 diabetes.”
International groups are developing guidelines and preparing for widespread adoption of CKM. Breakthrough T1D (formerly JDRF) Thomas Danne, M.D., PhD., Chief Medical Officer, International, said, “Experts from 12 countries agree: By detecting risk earlier and guiding faster action, CKM has the potential to reduce DKA, strengthen confidence for people with T1D, and transform how we deliver safe, proactive diabetes care.”
Seeing Danger Sooner: CKMs Matter
CKM shifts care from reactive (treating DKA) to proactive (preventing it). Ideally, CKM will integrate with glucose monitors and insulin pumps for comprehensive management.
Though CKM technology is still in development, researchers are conducting studies to refine device design, alarm thresholds, and clinical guidelines for its use.
These devices aim to help people with diabetes, especially type 1, providing real-time alerts to dangerously high ketone levels before they become a crisis, filling a significant gap in diabetes technology.


.webp)





.webp)
.jpg)

.jpg)

.jpg)



.jpg)

.jpeg)
.jpg)
.jpg)

.jpg)
.jpg)

.jpg)

.jpg)

.jpg)
.jpg)



.jpg)



.jpg)




.jpg)
.jpg)

.jpg)
.jpg)



.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)

.jpg)
.jpg)

.jpg)



.jpg)
.jpg)
.jpg)

.jpg)

.jpg)














.jpg)


.jpg)







.webp)


















.webp)